
|
|

|
|
|
|
|
Methods
About us
Request
News
Literature
Partners
© 2019, isolab® GmbH
Laboratorium für Stabilisotopenanalytik
Woelkestr. 9/I
D-85301 Schweitenkirchen
Germany
Telefon: +49 (8444) 918842
Fax: +49 (8444) 918844
Managing directors:
Dr. A. Roßmann
Amtsgericht: Ingolstadt HRB 10086
USt-Id-Nr.: DE 201083502
Imprint
AGB
Contact
Disclaimer
Privacy notice
Print this web page
Stable Isotopes´ Analytics for the Origin and Authenticity Control
of
food commodities, beverages, aromas, animal feed, wood and synthetic chemicals, and for the investigation of forensic and archaeological material

(Foto: Symrise GmbH & Co.KG, mit freundlicher Genehmigung Verlag Wiley-VCH, Weinheim)
Origin and authenticity of our food commodities determine their quality and price; it is a legal demand of the consumer that they correspond to each other. The determination of the stable isotopes´ ratios in bio-mass, water or mineral ingredients of food can control that he does not buy freshly squeezed fruit juice from concentrates, Bordeaux wine from Romania, Parmesan cheese made from Bavarian milk or German beef from Argentina.
How can such adulterations and fraudulences be recognized?
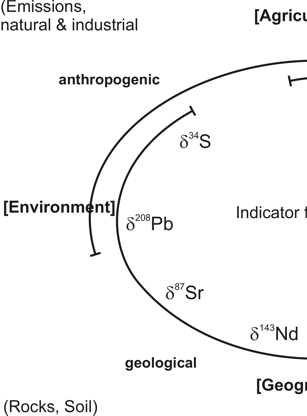
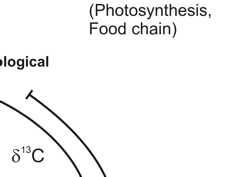
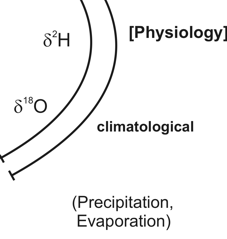
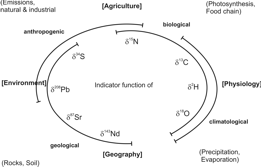
Any organic substance, hence also our food stuff, consists of the bio-elements H, C, N, O and S. Everywhere, these elements occur as mixtures of stable isotopes. The isotope ratios depend on those of local primary compounds, geological and climatic conditions and on anthropogenic influences at the site of the substances´ formation. Vice versa, it is possible to conclude from the isotope ratios of food commodities on their geographical origin and the conditions of their production. As the individual elemental isotope ratios react differently on these influences, they provide more or less independent but complementary information.
 |
 |
 |
 |
||
|
By courtesy of: dmz 21 18-23 (2003) |
||
Isotope ratios of samples in question are expressed relative to those of international standards in so-called δ-value scales, e.g. for carbon (standard V-PDB = Vienna PeeDee-Belemnite):
| δ13C[‰]V-PDB= |  |
(13C/12C)SAMPLE | -1 |  |
*1000 |
| ——————— | |||||
| (13C/12C)V-PDB |
δ13C-values ([‰]V-PDB) of bio-mass - photosynthesis type of plants
The carbon isotope effect on the primary reaction of the photosynthetic CO2-assimilation is responsible for the δ13C-value of the produced bio-mass. In the case of the so-called C3-plants, the most common of our indigenous plants, the δ13C-values range from -30 to -25‰, for the C4-plants (corn, sugar cane, sorghum) from -14 to -11‰, and for the CAM (crassulacean acid metabolism)-plants (pine apple, vanilla beans) from -21 to -18‰. On the basis of these characteristic values, the fraction of corresponding material in plant bio-mass or cattle feed can be determined. The indicated ranges of the above given values are caused by local climatic and physiological properties of the plants and imply corresponding additional information. Animal products reflect the diet with a trophic shift of ~1.5 ‰.
 To diagram
To diagram
δ15N-values ([‰]AIR) of bio-mass - nitrogen sources
The very primary source for organically bound nitrogen is air-N2 with δ15N = 0‰. N2 is the direct N-source for the nitrogen assimilation by legumes (used for "green fertilisation") and for synthetically produced ammonia and nitrate (used for mineral fertilisation). Nitrification, denitrification and ammonia evaporation lead to 15N-enrichments in the non-converted parts of the corresponding educts. As organic fertilisers and soil-bound nitrogen are normally enriched in 15N, products from organic production have often, but not exclusively, a more positive δ15N-value than those from conventional production. The trophic shift for animal products is ~+2‰ per level.
 To diagram
To diagram
δ2H- and δ18O-values ([‰]V-SMOW) of bio-mass - climate and water cycle
Water is an essential part of most food commodities; furthermore, it is the starting material for organically bound hydrogen and oxygen. Due to the higher vapour pressure of the "light" water molecules (1H1H16O) they accumulate in the vapour phase, whereas the "heavy" water molecules (1H2H16O, 1H1H18O) are concentrated in the condensates. Hence, meteoric water (precipitations and ground water) is the lighter, the farther from the ocean (continental effect) and the higher (altitude effect) it occurs. Furthermore, the local climate and plant anatomic factors influence the water evaporation from the plants and contribute to the isotope characteristics of the leaf water and from here to the δ18O/δ2H-values of the plant bio-mass. In animal bio-mass, the δ18O-value of water is composed by that of the drinking water, the diet and oxidation water. The δ2H-values of the main fractions from plant and animal tissues (carbohydrates, proteins, lipids) differ characteristically due to metabolic H-isotope fractions.
 To diagram
To diagram
δ34S-([‰]V-CDT) and δ87Sr-values of bio-mass - local geological peculiarities
δ34S-([‰]V-CDT) and δ87Sr-values of bio-mass are determined by the isotope characteristics of the local primary sources of these elements. These are, in the case of sulphur, besides soil and water SO42-, at the sea-side sea spray, an aerosol, and near industrial plants and highways SO2 from the combustion process. Hence, an origin assignment on the basis of δ34S-values demands corresponding authentic reference values. This is similar for 87Sr and for the isotopes of heavy metals like Pb, a human pollution product. Strontium is the most essential indicator for geographical origin assignments.
 To diagram
To diagram